Indian businesses have a golden opportunity in Vietnam’s fast-growing healthcare sector and demand for affordable medicines make its generic drug market. Known as the “pharmacy of the world,” Indian pharmaceutical companies like Sun Pharma and Dr. Reddy’s have the expertise to thrive in Vietnam’s $3 billion generic drug market, driven by a 100-million-strong population and rising health needs. But entering Vietnam requires navigating legal hurdles like licensing, taxes, and compliance.
At La Défense Law Firm, we help Indian businesses set up smoothly in Vietnam. This guide offers clear steps and legal tips for Indian firms to succeed in Vietnam’s generic drug market, making your journey easy and profitable.

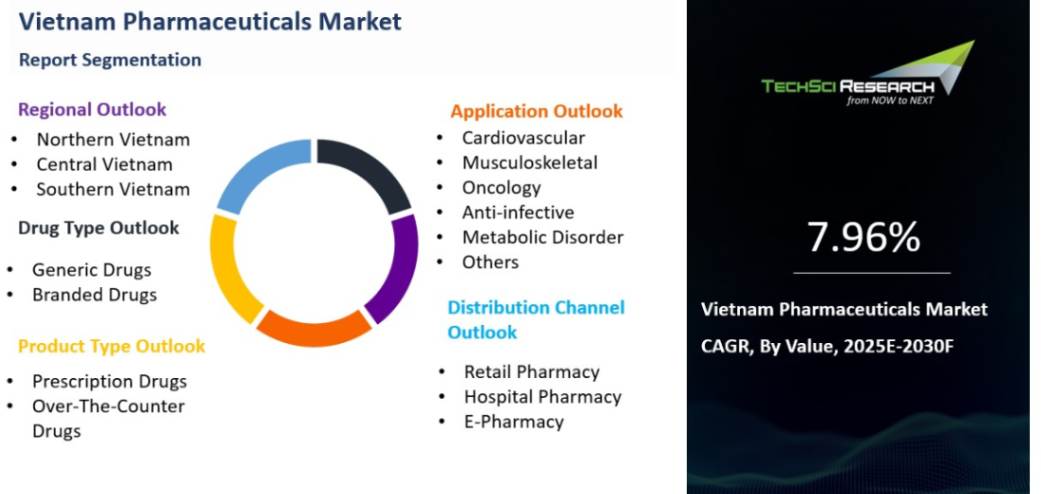
Why Vietnam’s Generic Drug Market Appeals to Indian Businesses
Vietnam’s generic drug market is booming, with a 7-9% annual growth rate. Hospitals and pharmacies in Hanoi, Ho Chi Minh City, and Da Nang need affordable drugs for conditions like diabetes or infections, where Indian generics excel. Vietnam imports 90% of its medicines, and Indian firms, known for low-cost, high-quality generics, can fill this gap. Plus, cultural ties—think Bollywood fans in Hanoi—make Indian brands relatable.
To start, understand Vietnam’s demand. La Défense Law Firm analyzes market needs, ensuring your generics, like antibiotics for Ho Chi Minh City clinics or heart drugs for Da Nang hospitals, meet local standards. We guide you through Vietnam’s health regulations, so your business connects with Vietnam’s growing healthcare scene.
Step 1: Register Your Business Legally
To operate in Vietnam, Indian businesses must register under the Law on Enterprises. You can set up a wholly foreign-owned company (like an LLC) or a joint venture with a local partner. Registration requires a Vietnamese application with your business plan, capital (often $50,000 minimum for pharma), and company details, submitted to the Department of Planning and Investment in Hanoi or Ho Chi Minh City. Mistakes, like unclear plans, delay approvals.
Legal help makes this simple. La Défense prepares your filings for a drug firm in Hanoi’s Ba Dinh or Ho Chi Minh City’s District 7. We ensure compliance, so your business—whether a factory in Binh Duong or an office in Nha Trang—starts strong, letting you focus on producing generics like paracetamol or metformin for Vietnam’s market.
Step 2: Secure Pharmaceutical Licenses
Vietnam’s Ministry of Health tightly regulates the generic drug industry. You need a Certificate of Pharmaceutical Business Eligibility, requiring a licensed pharmacist, proper facilities, and Good Manufacturing Practices (GMP) compliance. Each drug must be registered, with dossiers proving safety and quality, filed in Vietnamese. Without these, you can’t sell generics in Hanoi’s pharmacies or Da Nang’s clinics, and violations risk fines or bans.
Legal support saves time. La Défense guides Indian firms through licensing for plants in Hai Phong or labs in Ho Chi Minh City’s District 9. We compile dossiers and coordinate with the Ministry, ensuring your generics—say, anti-diabetics for Hanoi hospitals—meet Vietnam’s standards, getting you to market faster.
Step 3: Understand Tax Obligations
Vietnam’s taxes affect your profits. Corporate income tax is 20%, but pharma firms in industrial zones like Hanoi’s Long Bien or Binh Duong may get 10-17% rates for 10 years. VAT on drugs is 5-10%, and import duties apply to raw materials, though exemptions exist under the India-ASEAN Free Trade Agreement. Filing taxes in Vietnamese is mandatory, and errors, like missing deadlines, lead to audits.
Legal experts keep you compliant. La Défense files taxes for Indian businesses in Ho Chi Minh City’s District 1 or Da Nang, securing incentives for your generic drug plant. We handle Vietnamese forms, ensuring your firm—whether producing statins in Nha Trang or antibiotics in Hanoi—saves money while meeting Vietnam’s tax rules.
Step 4: Protect Your Intellectual Property
Indian generics rely on unique branding or processes, so protecting intellectual property (IP) is key in Vietnam’s competitive market. Register trademarks for your drug names or logos with the National Office of Intellectual Property, using Vietnamese filings. Without registration, copycats in Hanoi’s retail or Ho Chi Minh City’s online stores could harm your brand, like a fake version of your amoxicillin.
Legal services safeguard your IP. La Défense manages registrations for Indian firms, protecting generics in Da Nang’s markets or packaging in Hai Phong. We draft contracts to secure trade secrets, ensuring your business—whether a painkiller line in Hoi An or a vaccine in Binh Duong—stays safe and trusted in Vietnam.
Step 5: Hire and Train Staff Legally
Vietnam’s young workforce, with pharmacists and technicians in Hanoi and Ho Chi Minh City, is ideal for drug manufacturing. But the Labor Code requires work permits for Indian managers, with notarized degrees and experience, and local hires need Vietnamese contracts covering wages and social insurance (8% employee, 17.5% employer). Ignoring permits or contract rules risks fines or staff disputes.
Legal guidance ensures smooth hiring. La Défense secures permits for your team in Hanoi’s Cau Giay or Da Nang’s factories and drafts contracts for locals in Nha Trang. We align with Vietnam’s labor laws, so your generic drug plant—whether in Ho Chi Minh City’s District 7 or Bac Ninh—runs with a happy, legal workforce.
Step 6: Leverage Trade Agreements
The India-ASEAN Free Trade Agreement and Vietnam’s EU-Vietnam Free Trade Agreement (EVFTA) cut tariffs on drug exports, making Vietnam a hub for Indian generics to reach ASEAN or Europe. Ports in Hai Phong and Ho Chi Minh City ease shipping, but customs rules require Vietnamese compliance. Missing trade benefits or customs steps can raise costs or delay exports.
Legal support unlocks savings. La Défense helps Indian firms claim tariff exemptions for generics exported from Hanoi or Da Nang, ensuring customs compliance. We streamline processes, so your drugs—like antihypertensives for Europe or analgesics for ASEAN—flow smoothly from Vietnam’s ports to global markets.
Cultural and Legal Tips
Vietnam’s business culture values trust and patience. Rushing deals in Hanoi or skipping dinners with partners in Ho Chi Minh City can stall progress. Legal filings, like licenses or taxes, need Vietnamese precision, and bureaucracy in Da Nang or Nha Trang has its own pace. Indian businesses, used to fast-paced markets, might misstep without local insight.
Legal experts bridge this gap. La Défense coaches Indian firms on Vietnam’s norms, like respectful talks for factory deals in Hanoi’s Hoan Kiem or patient filings in Hoi An. We handle Vietnamese paperwork, ensuring your generic drug business—whether in Ho Chi Minh City’s District 3 or Hai Phong’s ports—fits Vietnam’s culture and laws.
Thrive in Vietnam’s Generic Drug Market
Vietnam’s generic drug market offers Indian businesses a chance to grow, with high demand, low costs, and trade perks. But legal hurdles—licensing, taxes, and IP—require careful steps to succeed. By partnering with experts, your firm can launch generics in Hanoi, Ho Chi Minh City, or Da Nang with confidence, meeting Vietnam’s health needs profitably.
La Défense Law Firm is here to guide Indian businesses into Vietnam’s drug market with clear legal support. Contact us today to start your journey and make a mark in this exciting industry.
Orther relevent legal articles:
- What You Need to Know: Rights and Obligations of the Accused and Defendant in Vietnam’s Criminal Proceedings in 2025
- Key Amendments in the 2015 Criminal Procedure Code of Vietnam: What’s Changed by 2025
- How Lawyers Make a Difference: Their Role in Vietnam’s Criminal Proceedings in 2025

